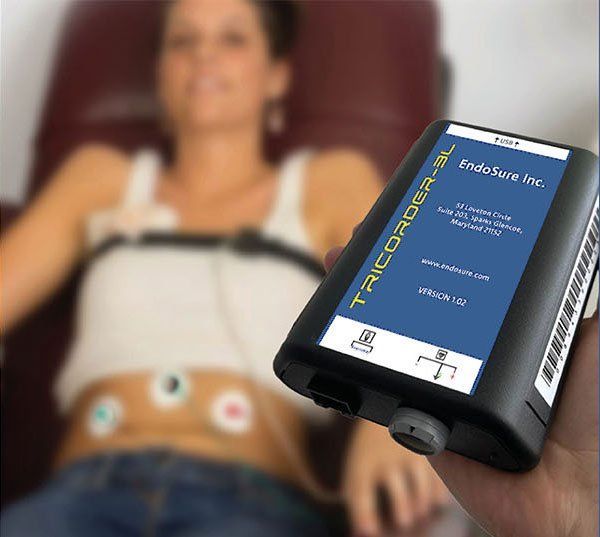
The Only Tier-1, Non-Invasive, 30-Minute, Highly Accurate Test with Instant Results to Facilitate the Diagnosis of Endometriosis.
Women worldwide suffer for an average of 8.6 years before being diagnosed with endometriosis.⁴ Leading the charge to change this, the EndoSure Test* takes 30 minutes to perform, and provides physicians with an easy to use, rapid diagnosis of endometriosis.
Endometriosis is a debilitating disease that effects over 10% of women globally. As a clinical decision support tool the
EndoSure Test* is the only noninvasive, 30-minute, objective test to help physicians quickly and accurately diagnose endometriosis regardless of patient age, disease stage or concurrent conditions. The test is a game changer in how physicians diagnose endometriosis, and can be used during the initial encounter for patients presenting with pelvic or menstrual pain and unprecedented opportunity for effective post-treatment monitoring regardless of endo treatment type.
Now Women Can Be Sure
Facilitating a diagnosis of endometriosis is now as easy as 1-2-3
The Only Non-Invasive, 30-Minute, Highly Accurate Diagnostic Endo Test That Detects the Presences of Endometriosis anywhere in the anatomy.² ³
The EndoSure Test* has a number of advantages: