FOR PRACTIONERS
The Global clinicians currently using the ENDOSURE TEST* call it a game changer! It is the only Non-Invasive, 30-Minute,¹ Highly Accurate Diagnostic Test that detects the presence of Endometriosis anywhere in the anatomy regardless of age, disease state or other concurrent diseases.
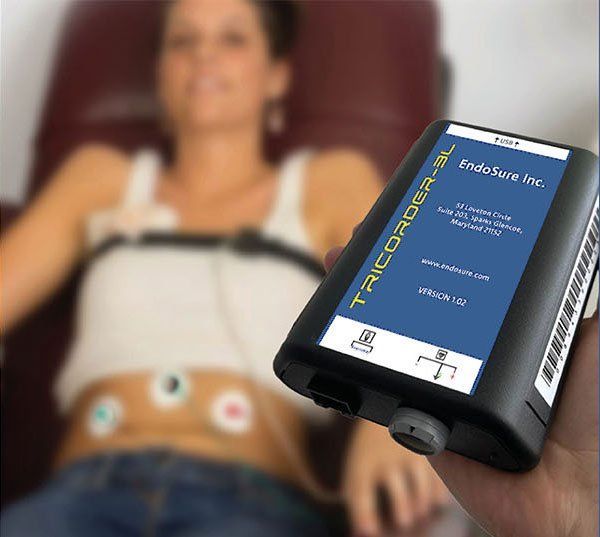
The endo test is also used to conduct post treatment follow-up regardless of treatment type.
The test can be performed during the initial encounter for patients presenting with pelvic or menstrual pain as well as other abdominal symptoms.
The EndoSure Test*
The endo test is easy to perform, and can be performed by a technician and provides definitive data in 30 minutes to confirm or eliminate the presence of endometriosis, whether in the abdominal/pelvic area as well as the chest, spine, brain or other anatomical regions.
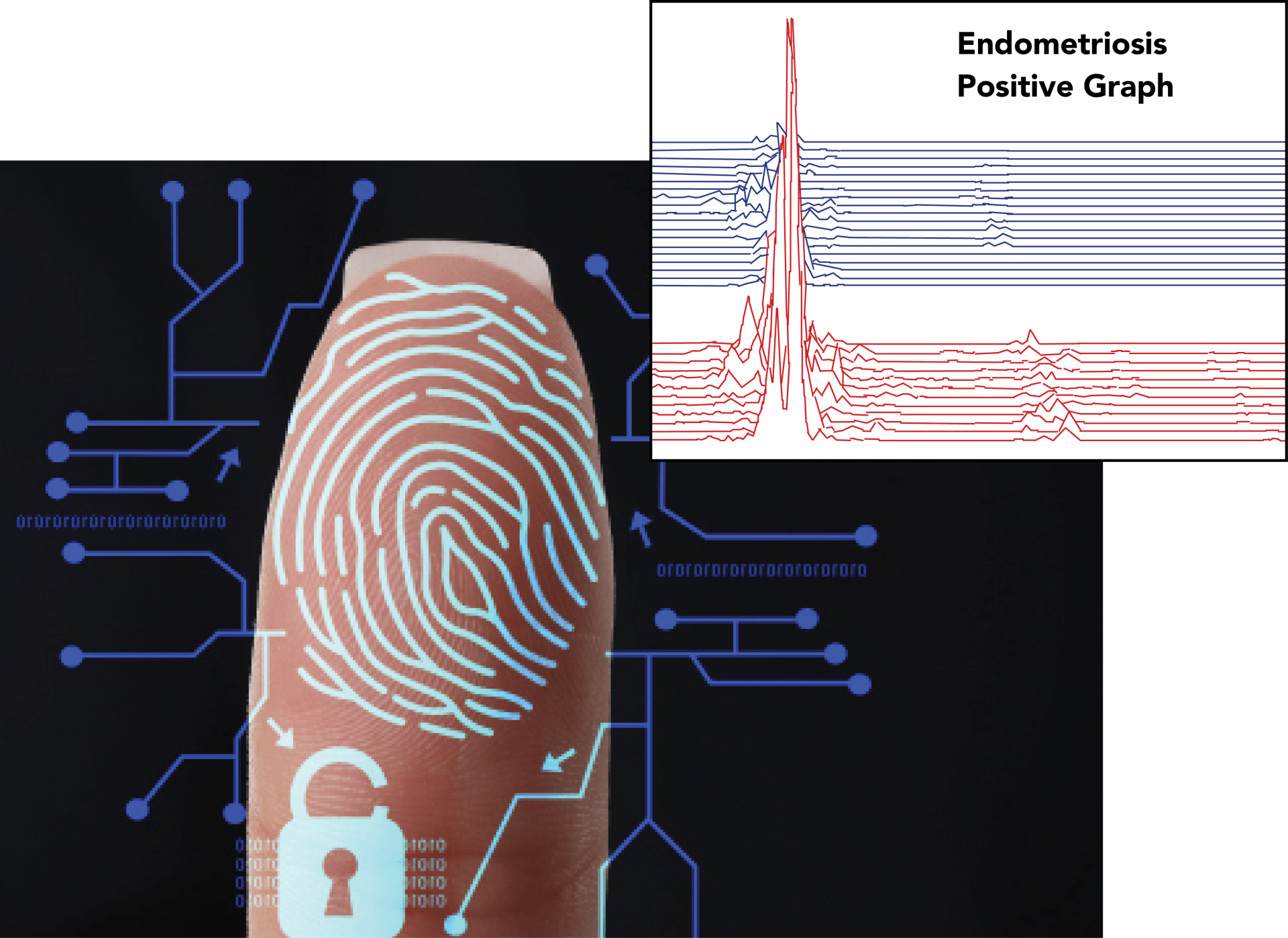
During the Electrogastrogram (EGG)/Electroviscerogram (EVG) portion of the test, the proprietary TRICORDER-3L collects data on very specific myoelectrical activity from the patient’s gastrointestinal tract. Research has shown that the neurotransmitters secreted by endometriosis tissue uncouple normal myoelectrical control in the gastrointestinal tract, resulting in idiosyncratic abnormally high frequency activity, producing a unique myoelectric signal.² Calculations, based on published research,³ are conducted by the EndoSure support software using this data. The resulting information can be used to support for rapid diagnosis of endometriosis during the first patient encounter.

